MINI-REVIEW
Klaus Mönkemüller, MD, PhD, FASGE, FESGE, FJGES
Professor of Medicine, Department of Gastroenterology, Carilion Memorial Hospital, Virginia Tech Carilion School of Medicine, Roanoke, USA
Correspondence: Klaus Mönkemüller, MD, PhD — Department of Gastroenterology, Carilion Memorial Hospital, Virginia Tech Carilion School of Medicine, Roanoke, VA, USA
Abstract
Background: Gastric neuroendocrine tumors (NETs), historically termed carcinoids, are an increasingly recognized entity encountered during routine endoscopy. Their classification into three distinct types with different etiologies, behaviors, and management strategies makes systematic understanding essential for the practicing endoscopist.
Methods and Results: We present a case of a 15 mm Type I gastric NET arising in the setting of autoimmune atrophic gastritis, successfully resected via endoscopic full-thickness resection (eFTR). A comprehensive review of the three-type classification system, endoscopic features and advanced imaging characteristics, WHO grading (G1–G2), and current management strategies is provided, illustrated with a 13-panel endoscopic and histologic image series.
Conclusion: Type I gastric NETs are the most common subtype and are generally amenable to endoscopic resection when <20 mm. Endoscopic full-thickness resection (eFTR) achieves R0 margins even for lesions extending to the muscularis propria. Systematic classification and laboratory workup allow appropriate risk stratification and avoidance of unnecessary surgery.
Keywords: gastric neuroendocrine tumor; gastric carcinoid; Type I NET; autoimmune atrophic gastritis; eFTR; endoscopic full-thickness resection; hypergastrinemia; WHO grading
★ Key Clinical Takeaways
- Type I gastric NETs (80% of cases) arise from chronic hypergastrinemia in autoimmune atrophic gastritis — not from a gastrinoma; distinguishing Type I from Type II requires clinical and laboratory correlation, not advanced endoscopic imaging alone.
- Advanced imaging (NBI/I-scan/FICE) cannot differentiate Type I from Type II, but is valuable for identifying additional small NETs missed on white light endoscopy.
- A solitary lesion >1 cm raises concern for Type III neuroendocrine carcinoma — the most aggressive subtype — and warrants aggressive staging and multidisciplinary workup.
- Endoscopic full-thickness resection (eFTR) is the preferred technique for lesions <20 mm, achieving R0 resection even when invasion extends to the muscularis propria.
Case Vignette
A 68-year-old woman with Hashimoto’s thyroiditis was referred for suspected “gastrinoma” (Zollinger–Ellison syndrome) with a gastrin level of 1000 pg/mL and a stomach “carcinoid.” The patient’s final diagnosis was chronic atrophic autoimmune gastritis and a Type I stomach neuroendocrine tumor (NET). This 15 mm lesion was resected using endoscopic full-thickness resection technique with an Ovesco eFTR device. The aim of this report is to provide a mini-review of gastric NETs or carcinoids with focus on their endoscopic features.
Classification of Gastric Neuroendocrine Tumors
Gastric NETs are classified into three types:
- Type 1 (80% of cases): Associated with autoimmune atrophic gastritis, pernicious anemia, and B12 deficiency.
- Type 2: Associated with Zollinger–Ellison syndrome and hypergastrinemia from a gastrinoma.
- Type 3: Neuroendocrine carcinoma — usually solitary, >1 cm, and the most dangerous.
Both Type 1 and Type 2 are associated with hypergastrinemia but via different mechanisms. In Type 1, the increased gastrin results from autoimmune atrophic gastritis; in Type 2, it is produced by a tumor (gastrinoma). Type 1 and 2 gastric NETs are usually well-differentiated with a low Ki-67 index, multiple, and <1 cm. A solitary gastric NET is more concerning for Type 3, while multiple lesions suggest Type 1 or 2.
Diagnosis and Workup
Differentiation between Type 1 and Type 2 relies on clinical features, endoscopic appearance, and laboratory tests (gastrin levels, anti-parietal cell antibodies, etc.). NBI/I-scan/FICE cannot differentiate between Type 1 and Type 2, but advanced imaging endoscopy is useful for identifying additional small NETs missed on white light endoscopy. The typical endoscopic characteristics of gastric NETs include their sessile shape, flat top, yellowish color, and hypervascularity (see Figure 1).
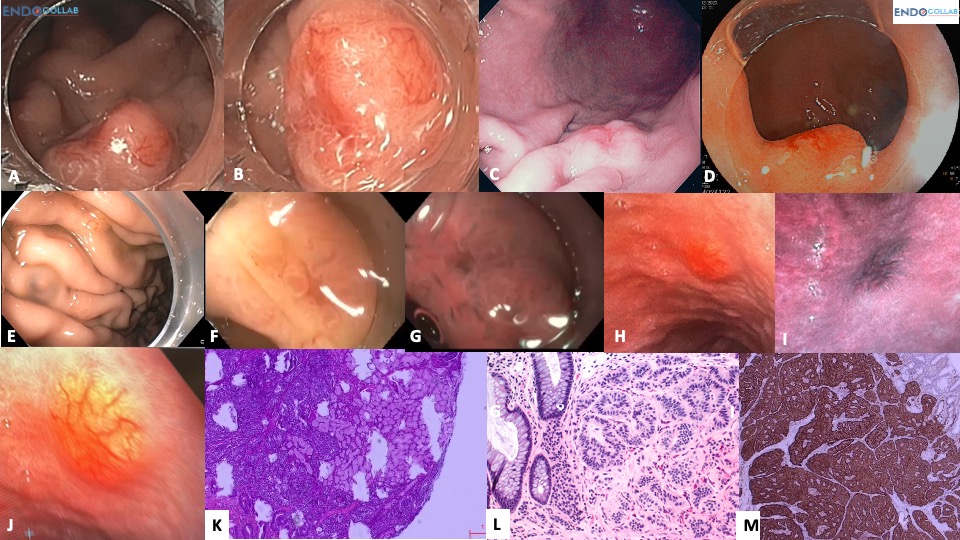
WHO Grading
- Grade 1 (G1): <2 mitoses/10 HPF; Ki-67 <3%.
- Grade 2 (G2): 2–20 mitoses/10 HPF; Ki-67 3–20%.
Endoscopic Management
Smaller carcinoids (<20 mm) can generally be managed by endoscopic resection. Resection should be aggressive and aim for R0. The best methods are endoscopic full-thickness resection (eFTR) or ESD with intermuscular dissection, as some lesions extend as deep as the muscularis propria.
List of Abbreviations
NET: neuroendocrine tumor; eFTR: endoscopic full-thickness resection; ESD: endoscopic submucosal dissection; NBI: narrow-band imaging; FICE: flexible intelligent chromoendoscopy; Ki-67: proliferation marker index; HPF: high-power field; PET: positron emission tomography; G1: Grade 1; G2: Grade 2; WHO: World Health Organization
References
- Nakamura S, Iida M, Yao T, Fujishima M. Endoscopic features of gastric carcinoids. Gastrointest Endosc. 1991 Sep–Oct;37(5):535–8. doi: 10.1016/s0016-5107(91)70823-7. PMID: 1936831.
- Hülagü S, Yilmaz H. Gastric carcinoid tumors. Ann Clin Exp Metabol. 2017;2:1017.
- Chuah SK, Hu TH, Kuo CM, et al. Upper gastrointestinal carcinoid tumors incidentally found by endoscopic examinations. World J Gastroenterol. 2005 Nov 28;11(44):7028–32. doi: 10.3748/wjg.v11.i44.7028. PMID: 16437611.
Patient Consent
Written informed consent was obtained from the patient prior to publication of this case and associated images.
Funding
This work received no external funding.
Conflict of Interest
The author declares no conflict of interest.

